Abstract
Introduction: The bortezomib, lenalidomide, and dexamethasone (VRd) regimen is an acceptable standard of care (SoC) for both transplant-eligible and transplant-ineligible newly diagnosed multiple myeloma (TI NDMM). Ongoing development of novel therapies and combinations strive to improve survival outcomes beyond what is expected from SoC. Belantamab mafodotin (belamaf) is a B-cell maturation antigen-binding antibody-drug conjugate that eliminates myeloma cells by a multimodal mechanism and has demonstrated durable responses in patients with relapsed/refractory multiple myeloma (RRMM). Preclinical evidence of belamaf in combination with bortezomib or lenalidomide suggests enhanced anti-myeloma activity, providing rationale for this treatment combination. We report the preliminary findings of belamaf + VRd for TI NDMM patients.
Methods: DREAMM-9 (NCT04091126) is an ongoing Phase I, open-label, randomized, dose and schedule evaluation study of belamaf + VRd in patients with TI NDMM. Eligible patients include those ≥18 years old with ECOG status 0-2 and adequate organ system functions.
The study evaluates safety and tolerability of belamaf + VRd in up to 8 cohorts, up to 144 patients, to establish the recommended phase 3 dose (RP3D). VRd is administered Q3W until cycle 8, followed by lenalidomide + dexamethasone (Rd) Q4W. Belamaf + VRd is administered until cycle 8, and then in combination with Rd thereafter. The belamaf dose cohorts currently being evaluated are: cohort 1 (1.9 mg/kg Q3/4W), cohort 2 (1.4 mg/kg Q6/8W), cohort 3 (1.9 mg/kg Q6/8W), cohort 4 (1.0 mg/kg Q3/4W), and cohort 5 (1.4 mg/kg Q3/4W). After evaluation of safety data for cohort 1, cohorts 2-5 were opened in parallel and enrolled patients were randomized 1:1:1:1. Safety data, clinical activity, and drug concentrations will be assessed, and used to determine the belamaf RP3D. This analysis reports the preliminary results from cohort 1.
Primary endpoints include number of patients with adverse events (AEs). Secondary endpoints include establishing relative dose intensity of lenalidomide and bortezomib in combination with belamaf, cumulative dose of belamaf, pharmacokinetics (PK) profile of belamaf when combined with VRd, overall response rate (ORR), complete response (CR), stringent complete response (sCR), complete response rate ([CRR]; % of patients with a confirmed CR or better), and rate of very good partial response or better (≥VGPR). Exploratory endpoints include assessing minimal residual disease (MRD) in patients who achieve ≥VGPR, and safety and efficacy exposure-response relationships.
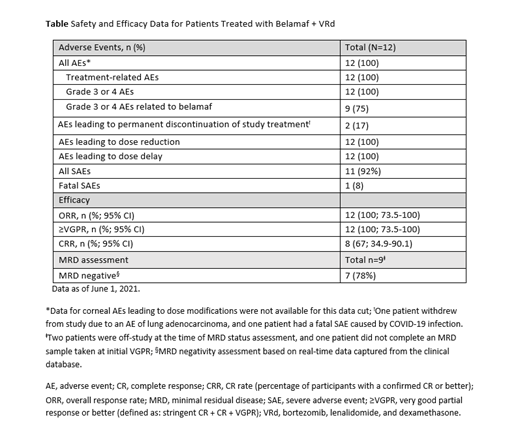
Results: Twelve patients in cohort 1 were included in this preliminary analysis. Eight patients (67%) were male; median age (range) was 72.5 years (63-77). Ten patients (83%) were white and 2 (17%) were Asian. Nine patients (75%), were ISS stage II or III, and 4 (33%) patients had high-risk cytogenetics (consisting of one or more of the following: t(4;14), t(14;16), del17p, 17p13del).
AEs related to study treatment were experienced by all 12 patients. Dose reductions occurred in 12 (100%) patients, all of whom also experienced a dose delay. Most common AEs leading to dose modification were thrombocytopenia, neutropenia, and corneal events. Grade 3 or 4 AEs related to belamaf occurred in 9 (75%) patients. During the trial, one patient experienced a fatal severe AE due to COVID-19 infection (unrelated to study treatment; Table).
All patients, 100% (n=12; 95% CI: 73.5-100) achieved ≥VGPR. Early deep responses were observed; 2 (17%) patients achieved VGPR as early as 4 weeks. As of data cut-off, 5 (42%) remain in CR and 3 (25%) in sCR. Based on real-time data captured in the clinical database, 7 out of 9 patients achieved MRD-negative status at the first test after VGPR.
Belamaf PK profile was similar to that observed in patients with RRMM taking into consideration baseline patients characteristics.
Conclusion: Preliminary data suggest addition of belamaf to VRd did not reveal new safety signals and demonstrates high response rates, albeit with short follow-up. The study is ongoing to confirm safety and evaluate the efficacy of belamaf + VRd. Updated data for cohort 1 will be reported at the congress.
Funding: GSK (Study 209664); belamaf drug linker technology licensed from Seagen; belamaf monoclonal antibody produced using POTELLIGENT Technology licensed from BioWa.
Usmani: Pharmacyclics: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Takeda: Consultancy, Research Funding, Speakers Bureau; Merck: Consultancy, Research Funding; SkylineDX: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Janssen Oncology: Consultancy, Research Funding; Bristol-Myers Squibb: Research Funding; EdoPharma: Consultancy; GSK: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Array BioPharma: Consultancy, Research Funding; Abbvie: Consultancy; Amgen: Consultancy, Research Funding, Speakers Bureau. Quach: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen/Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Antengene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; CSL: Consultancy, Membership on an entity's Board of Directors or advisory committees. Koh: Pfizer: Consultancy; Jassen: Honoraria; AstraZeneca: Honoraria; Novartis: Honoraria; GSK: Honoraria; Roche: Honoraria; Takeda: Honoraria. Guenther: Novartis: Consultancy; Celgene: Consultancy, Honoraria; Roche: Consultancy; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; AbbVie: Consultancy; Jazz Pharmaceuticals: Honoraria; Janssen Pharmaceuticals: Consultancy, Honoraria. Zhou: GlaxoSmithKline: Current Employment. Kaisermann: GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company. Mis: GlaxoSmithKline: Current Employment. Williams: GlaxoSmithKline: Current Employment. Yeakey: GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company. Ferron-Brady: GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company. Figueroa: GlaxoSmithKline: Current Employment. Kremer: GlaxoSmithKline: Current Employment. Gupta: Novartis: Current equity holder in publicly-traded company; GlaxoSmithKline: Current Employment, Current equity holder in publicly-traded company. Janowski: Celgene: Consultancy; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal